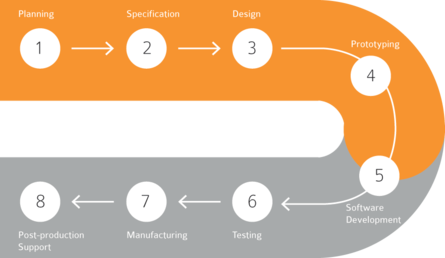
Implementation of safety systems with high quality and minimum business interruptions. jnr's highly experienced team members made highly complex implementations done in quality and timely manner.We help the organizations to manage drug safety application more accurately and confidently.We offer business process re-engineering, configuration, data migration, validation, integration, report development, training, go-live and post implementation support.We follow a phased approach to implementing Argus Safety system:
- Phase 1: Project Scoping
- Phase 2: Requirement Gathering and Analysis
- Phase 3: Implementation of Argus Safety
- Phase 4: Post Production Support.

We offer out-of-the-box as well as complete warehousing solution to our customers leveraging Business ObjectsTM, IBM CognosTM, OBIEE, Qlikview, Spotfire, Big Data, SAS.DashboardRegulatory reports – PSUR/DSUR, PBRER, CTPR, NDA, IND, PADERCompliance monitoringSignal detection & risk managementSafety surveillance and product quality reportsLine listingsProductivity and Cost Analysis
JNR Consulting Group with its proven data migration methodology and extensively experienced consultants can help pharmaceutical companies to reduce delivery timelines and implementation cost without compromising on data quality. Our consultants have hands on experience of 20+ drug safety data migrations with impressive and proven track record.

Approach
- Analyze – Plan and decide with business to migrate what is required and feasible.
- Profile –Perform data profiling to uncover quality issues & make informed decisions to ensure high data quality.
- Design – Collaborate with the business community and create mapping specification & validation documents.
- Develop – Customize data migration engine according to mapping specification
- Migrate –Perform migration with minimum business downtime
- Validate – Validate migrated data for all possible scenarios
- Deliver – Deliver everything with high qualityOur team’s migration experience:ARISg/ARISj to Argus/ArgusJArgus to ARISgArgus to ArgusOracle AERS to ArgusArgus to Oracle AERSPerceive to ArgusJEmperica Trace to ArgusHome grown systems to ArgusExcel, flat files, XML.
Our team with in-depth knowledge of Oracle® Argus Safety™ Suite and ArisGlobal® ARISg is provided 24×7 functional and technical support to our customer in daily case processing, E2B reporting and periodic report authoring.
Application User TrainingOur Argus SafetyTM consultants have conducted Application User Trainings (online and classroom training) for various large and mid-size pharmaceutical and bio-technology companies. This included Argus Affiliate, case intake, case processing, reporting, configuration and generation of periodic reports and configuration of product, licenses and studies.

Need to Discuss with Us
Our helpline is always open to receive any inquiry or feedback. Please feel free to drop us an email from the form below and we will get back to you as soon as we can.